Overview
Definition of terms
| Term | Definition |
|---|---|
| Osmosis | Spontaneous movement of water across a semi-permeable membrane from a region of low solute concentration to one of high solute concentration which tends to equalize the solute concentration on either side of the membrane |
| Osmotic pressure | The hydrostatic pressure necessary to counteract the process of osmosis |
| Osmolarity | The total number of solute particles per volume of solution |
| Osmotic gradient | The difference in osmolarity of two solutions on either side of a semi-permeable membrane |
| Tonicity | A measure of the effective osmotic gradient between two fluids separated by a semi-permeable membrane. It is influenced only by solute particles that cannot cross the membrane. IV fluids can be classified as Isotonic, Hypertonic, and Hypotonic relative to the intracellular space. |
| Fluid Resuscitation | Replacement of fluid in hypovolemic patients |
| Fluid Maintenance | Replacement of ongoing physiologic fluid losses e.g. urine production roughly in real-time |
| Balanced solution | IV fluid that closely approximates the electrolyte concentration of normal plasma e.g. RL, Hartmann’s |
Osmolarity vs Tonicity
| Osmolarity | Tonicity |
|---|---|
| Absolute value | Relative value compared with the intracellular space |
| Dependent upon all particles of a solute | Dependent upon particles that exert an osmotic force |
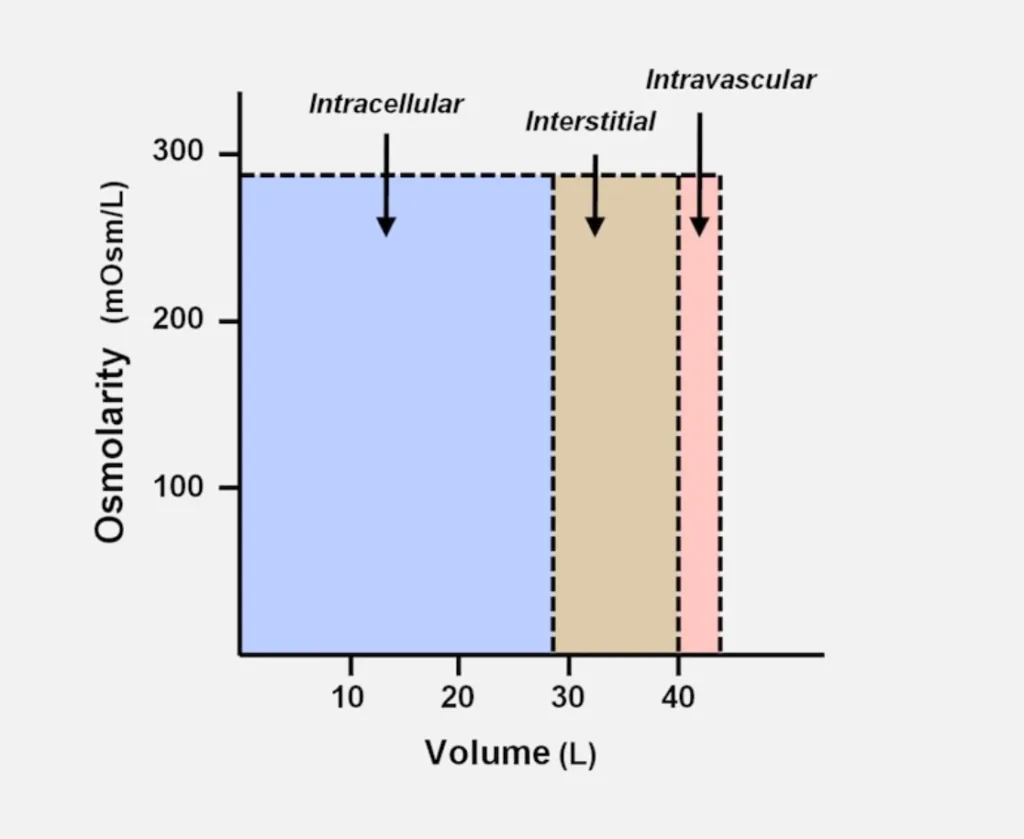
Body Fluid Compartments
Men: Total Body Water = weight x 60% (about 4.2 Litres in a 70kg man)
Women: Total Body Water = weight x 50%
5 – 15 – 40 rule: 5% weight is intravascular water (plasma), 15% is interstitial, 40% is intracellular ****
The 3 main fluid compartments are in the ratio of 8:3:1 (40 – 15 – 5 rule)
2/3 (40%) of total body water(TBW) is intracellular fluid.
1/3 (20%) of TBW is extracellular fluid.
3/12 (15%) is Interstitial fluid.
1/12 (5%) of TBW is plasma.
Physiological Regulation of Extracellular Fluid Volume
| Hormone | Effect |
|---|---|
| Aldosterone | Enhances sodium reabsorption, Increases intravascular volume |
| Antidiuretic hormone (Vasopressin) | Enhances water reabsorption |
| Atrial Natriuretic Peptide | Enhances sodium and water excretion |
Effect of IV fluids on Fluid compartments
The greater the tonicity of IV fluid ([NaCl]), the less fluid is required to expand the extravascular compartment to a specific fluid. Infusion of non-isotonic fluids will affect the body’s osmolarity.
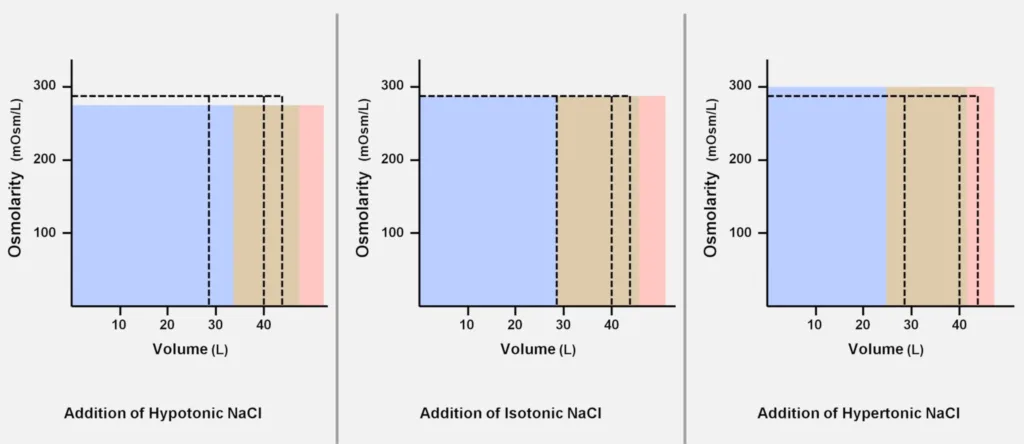
Isotonic fluid: Does not change the intracellular volume. Is distributed equally in the extracellular compartment.
Hypotonic fluid: Expands the intracellular and extracellular compartments. A larger volume is needed to expand the intravascular volume.
Hypertonic fluid: Reduces the intracellular compartment and pulls fluid into the extracellular compartment. Less fluid is needed to expand the intravascular volume. The increase in plasma osmolarity can lead to CNS dysfunction.
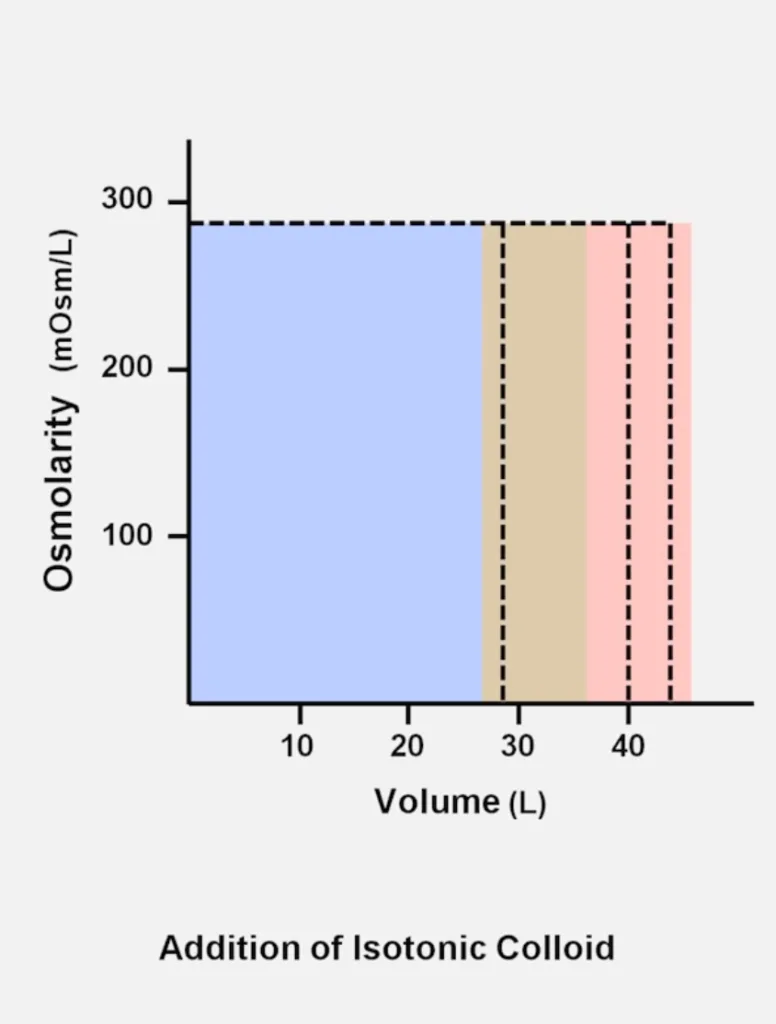
Colloid: Reduces the intracellular and interstitial compartments, but rapidly expands the intravascular compartment. Even less amount of colloid is needed to raise intravascular volume.
Evaluation of Intravascular Volume
Pre-operative evaluation
- History
- Hypovolemia: Vomiting, diarrhoea, fever, sepsis, trauma
- Volume overload: weight gain, edema, acute renal failure, ascites
- Physical exam
- Hypovolemia: skin tugor, capillary refil, dry mucous membrane, tachycarida, orthostasis, decreased urine output
- Volume overload: pitting oedema, crackles, wheezing, elevated jugular venous pressure
- Investigations
- Hypovolemia: Hematocrit, contraction alkalosis → metabolic acidosis, urine specific gravity > 1.010, hypernatremia, BUN:Cr > 10:1, bedside ultrasound of IVC <1.7 cm OR 1.7 cm with >50% IVCCI
- Volume overload: increased pulmonary vascular markings on chest x-ray, B-lines on lung ultrasound
Intra-operative evaluation
- Vitals
- Heart Rate and Blood Pressure trends (consider the impact of PPV and anaesthetics)
- Pulse oximetry waveform changes from baseline (assuming patient is normothermic and not in shock. A pulse pleth variability index (PVI) >12-16% is volume responsive
- Foley catheter
- Urine output: ADH levels may be increased due to stress response. This is a less reliable measure of volume status intra-operative
- Arterial line
- Serial Arterial Blood Gas (pH, Hct, electrolytes)
- Pulse pressure variation (PPV): indicates preload responsiveness (a small fluid challenge). A PPV > 10% suggests the patient is volume responsive. Not reliable if the patient is not in sinus rhythm, the chest is open, or not on PPV, or if Tidal Volume is > 8ml/kg.
- Central venous catheter
- Absolute Central Venous Pressure (CVP) measurement: unreliable, but trends can be meaningful
- Pulmonary artery catheter
- Used in RV dysfunction, pulmonary hypertension, valvular pathology and LV dysfunction
- Transesophageal echocardiogram
- Commonly used in major cardiac surgery and liver transplant. Most accurate assessment of volume status in the transgastric view. Can be used to narrow down the differentials of hemodynamic instability.
Crystalloid Fluids
Electrolyte solutions with a low tendency to stay intravascular.
| Fluid | Na+ mEq/l | Cl- mEq/L | K+ mEq/L | Ca2+ mEq/L | Glucose g/L | Buffer | Osmolarity | Tonicity | Indication |
|---|---|---|---|---|---|---|---|---|---|
| Normal Plasma | 140 | 100 | 4 | 2.4 | 0.85 | HCO3- 24mEq/L | 290 | N/A | N/A |
| 0.9% Saline (NS) | 154 | 154 | 0 | 0 | 0 | 0 | 308 | Isotonic | Resuscitation |
| 0.45% Saline (1/2 NS) | 77 | 77 | 0 | 0 | 0 | 0 | 154 | Hypotonic | Maintenance |
| 3% Saline | 513 | 512 | 0 | 0 | 0 | 0 | 1026 | Hypertonic | Severe Hyponatremia |
| D5 1/2 NS + 20 meq KCL | 77 | 97 | 20 | 0 | 50 | 0 | 446 | Hypertonic → Isotonic | |
| RL and Harmann’s | 130 | 109 | 4 | 3 | 0 | Lactate 28 mEq/L | 273 |
0.9% Saline (NS)
Isotonic fluid. Even though its osmolarity is slightly higher than normal plasma, it does not act as an ideal solution and its empirically measured osmolarity is much closer to the plasma than might be assumed by adding together the osmolarity of Na+ and Cl-.
- Indications
- Resuscitation
- Preferred in brain injury/swelling (hyperosmolar)
- Preferred for diluting pRBCs
- Downsides
- Dilutional acidosis (Hyperchloremic metabolic acidosis) – Normal anion gap metabolic acidosis when large volumes of normal saline are used (bicarbonate is diluted as CO2 remains the same). Mild in severity, modest clinical implication.
- Hyperchloremic → low GFR and risk of Acute Kidney injury
0.45% saline (1/2 NS)
Hypotonic fluid.
- Indication
- Maintenance
3% Saline
Hypertonic fluid. Not great for rapid intravascular expansion.
- Indications
- Severe Hyponatremia (to rapidly increase plasma osmolarity)
D5 1/2 NS + 20mEq/L KCL
The predicted tonicity is hypertonic. However, glucose is rapidly taken up by cells leaving the fluid isotonic.
- Indications
- Maintenance
Ringer’s Lactate (RL) and Hartmann’s Solution
Isotonic. The terms RL and Hartmann’s solution can be used interchangeably. However, there are small variabilities in the reported electrolyte concentrations. RL was the first balanced solution and is unique due to the anionic metabolizable buffer Lactate.
- Indications
- Resuscitation (more physiologic ‘balanced’ and lactate is converted to HCO3- by the liver)
- Maintenance (less frequently)
- Why would lactate be intentionally added to RL when it may cause anion gap metabolic acidosis (in addition to its use as a marker for poor tissue perfusion in shock)?
- Lactate is metabolized by the liver into bicarbonate providing a buffer to help prevent the acid-base disturbances seen with rapid infusion of normal saline.
- Contraindicated
- Hyperkalemia (due to presence of K+, minimal concern)
- Concurrent blood tranfusion (Ca2+ in RL can bind to citrate in blood products)
Electrolyte-Free Water
Various concentrations of sugar water. Fully distributes to all 3 fluid compartments and is thus not a good choice to expand extracellular volume. Only used in situations of persistent hypoglycemia or to bring down serum osmolarity when it is elevated. Examples include D5W and D10W
| Fluid | Na+ mEq/l | Cl- mEq/L | K+ mEq/L | Ca2+ mEq/L | Glucose g/L | Buffer | Osmolarity | Tonicity | Indication |
|---|---|---|---|---|---|---|---|---|---|
| Normal Plasma | 140 | 100 | 4 | 2.4 | 0.85 | HCO3- 24mEq/L | 290 | N/A | N/A |
| D5W | 0 | 0 | 0 | 0 | 50 | 0 | 446 | Isotonic | Hypernatremia, Hypoglycemia |
D5W
Hypotonic fluid. The infused glucose is rapidly taken up by cells making it hypotonic.
- Indications
- Hypernatremia
- Hypoglycemia
Colloids
Solution with large molecules that have a high tendency to stay intravascular. Volume expansion due to colloids is determined by their molecular weight and concentration.
| Colloid | Example |
|---|---|
| Natural colloids | Albumin, FFP, Whole blood |
| Synthetic colloids | Dextrans, hydroxyethyl starch, gelatins |
| Fluid | Avg Molecular Wight (kD) | Oncotic pressure (mmHg) | Initial Volume Expansion | Duration of Volume Expansion |
|---|---|---|---|---|
| 4-5% Albumin | 69 | 20-30 | 70-100% | 12-24 hrs |
| 20-25% Albumin (High-concentration albumin) | 69 | 70-100 | 300-500% | 12-24 hrs |
| 10% Dextran 40 | 40 | 20-60 | 100-200% | 1-2 hrs |
| 6% hydroxyethyl starch (Hespan) | 450 | 25-50 | 100-200% | 8-36 hrs |
- Indications
- Resuscitation in severe hypovolemia: if expecting to give 3-4 L of crystalloid for resuscitation, or crystalloid resuscitation is inadequate. 1/2 life is 3-6 hours vs 20-30 minutes for crystalloids
- Patients with large protein losses and decreased oncotic pressure e.g. burns, cirrhotic patients
- Fluid resuscitation in hemorrhagic shock when blood is not initially available (1cc colloid for every cc of blood lost)
- Albumin is used in cirrhotic patients
- Treatment protocol for spontaneous bacterial peritonitis (High-volume albumin to prevent hepatorenal syndrome)
- Large volume Paracentesis (High-concentration albumin)
- Renal failure of undetermined etiology, but decreased renal perfusion from hypovolemia is a posibility
- Major downsides of synthetic colloids
- High-cost
- Allergic reactions and anaphylaxis
- Coagulation abnormalities (newer starch formulations – tetrastarches – are less likely to cause coagulopathy and anaphylaxis)
- Renal failure
Albumin (5% and 25%)
Derived from pooled donated blood after cold ethanol extraction and ultra-filtration. 5% albumin is used for hypovolemia, while 25% is used for hypovolemia in patients with restricted fluid and Na intake.
Hetastarch (6% hydroxyethyl starch, Hespan)
Solution of highly branched glucose chains. Degraded by amylase and eliminated by the kidney.
Max dose: 15-20 ml/kg/day
- Side effects
- Can increase PTT (via factor VIII/vWF inhibition) and clotting times
- Anaphylaxis: wheezing and urticaria
- Interferes with platelet function
- Contraindications
- Coagulopathy
- Heart failure
- Renal failure
Fluid Resuscitation
Fluid resuscitation is the rapid delivery of fluid to patients who have acutely impaired hemodynamics. Patients with hypovolemic shock, dehydration, severe sepsis, and septic shock are universally given IV resuscitation fluids.
- When to give fluid resuscitation (generally…)
- MAP < 60-65 mmHg and/or CVP < 8 mmHg
- No evidence of cardiogenic pulmonary edema
Fluid responsiveness
To decide when to use IV fluids ask: Is this patient fluid responsive? Fluid responsive means that the patient’s O2 delivery to peripheral tissue will improve after being given IV fluids. It is present when stroke volume or cardiac output increases by ≥ 15% after receiving a 500mL bolus of IV fluid.
- Dynamic tests of fluid responsiveness in spontaneously breathing patients
- Increased pulse pressure in response to passive leg raise
- Collapsibility of IVC with inspiration on bedside ultrasound
- Dynamic tests of fluid responsiveness in sedated and mechanically ventilated patient
- Pulse pressure variation
- Stroke volume variation
- Aortic flow velocity
Selection of IV fluids in resuscitation
- Crystalloids vs Colloids
- In general, colloids offer no benefit over crystalloids for resuscitation
- Crystalloids are preferred due to their decreased cost and availability
- Hydroxyethyl starch is contraindicated in septic shock due to increased mortality and increased risk of AKI
- Normal Saline vs Balanced Solutions (e.g. RL) Physicians prefer saline while surgeons and intensivists prefer balanced solutions
- Balanced solutions have a theoretical physiologic advantage over Normal Saline
- Using normal saline for resuscitation is still acceptable since there is lack of conclusive evidence of harm
- Normal saline leads to a normal anion gap metabolic acidosis of uncertain significance
- Normal saline may increase the risk of renal dysfunction in patients with shock due to renal vasoconstriction
| Advantages | Disadvantages | |
|---|---|---|
| Crystalloids | Lower cost, Readily available | Requires more volume for the same hemodynamic effect, Short IV t1/2 (20-30min), Dilutes plasma proteins → peripheral/pulmonary oedema |
| Colloids | Replace IV volume and hemodynamics with less volume and in less time, Longer IV t1/2, Maintains oncotic pressure, Less cerebral edema in healthy brain tissue, less intestinal oedema | Expensive, coagulopathy (dextran > HES), potential renal complications, may cause cerebral edema in injured brain, possible anaphylaxis |
Administering IV Fluids for resuscitation
IV fluids are given as boluses in resuscitation. After each bolus, the patient should be reassessed q2min until the shock is resolved. Poiseuille’s Law states that the resistance of fluid flow through a tube is proportional to the length of the tube and inversely proportional to the radius fourth power (Small changes to lumen width make huge changes to resistance). The best choice for the IV line is short and large-bore (and the worst is long and thin). Crystalloids are normally given in a ratio of 3:1 (3000ml for every 1000mL lost) and colloids are given in a ratio of 1:1.
- Bolus for previously healthy patients
- 1-2 L at a time
- Bolus for patients with mild-moderate CHF or ESRD
- 500-1000 mL at a time
- Bolus for patients with severe CHF or ESRD
- 250mL at a time
- Bolus for patients in septic shock
- 30mL/Kg crystalloids as an “initial fluid challenge” (Recommended by The Surviving Sepsis Campaign)
Evidence that a patient is Adequately Resuscitated
- MAP ≥ 65 mmHg
- CVP 8-12 mmHg
- Urine output ≥ 0.5 mL/kg/h
- ScvO2 ≥ 70%
- Normalized lactate if elevated, to begin with (<2mmol/L)
- Normalized mental status if abnormal to begin with
Some studies have found that CVP is a poor predictor of fluid responsiveness.
A patient’s heart rate is not a reliable indicator of volume status since multiple factors, in addition to hypovolemia, can lead to tachycardia e.g. pain, anxiety, medication side-effects, CHF, arrhythmia. Patients can also fail to mount a tachycardic response to hypovolemia due to B-blockers and Non-dihydropyridine CCBs.
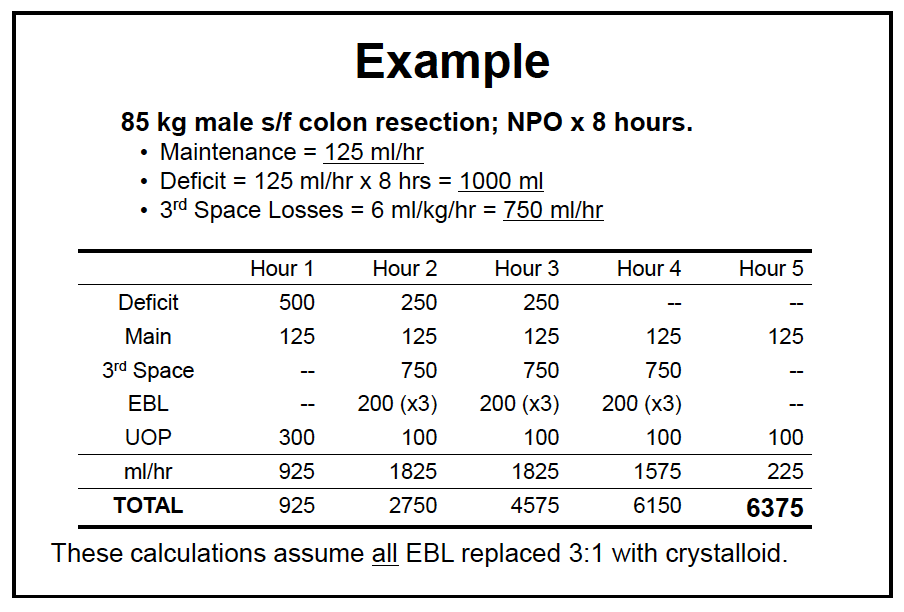
Classical Fluid Management
- Maintenance
- 4 – 2 – 1 rule: 4ml/kg/hr for the 1st 10kg, 2ml/kg/h for the next 10-20 kg, and 1 ml/kg/hr for each additional kg above 20kg
- Simply put, if the patient is > 20kg: maintenance = 40 + weight
- Pre-existing fluid deficit
- Multiply maintenance requirement by number of hours NPO
- Give 1/2 over 1st hour, 1/4 over 2nd hour, and 1/4 over 3rd hour
- Patients no longer undergo bowel preparation so deficit is decreased
- Ongoing losses – Evaporative and Interstitial Losses (capillary leak)
- Minimal tissue trauma e.g. hernia repair): 0 – 2 ml/kg/h
- Moderate tissue trauma e.g. cholecystectomy: 2-4 ml/kg/h
- Severe tissue trauma e.g. bowel resection: 4 – 8 ml/kg/h
- Estimating and Replacing Blood Loss
- Approximating Estimated Blood Loss (EBL) = (suction canister – irrigation) + ‘laps’ (100 – 150 ml each) + 4x 4 sponges (10 ml each) + field estimate
- Replace EBL with pRBCs, colloid, or crystalloids
Maximum Allowable Blood Loss (MABL)
The maximum allowable blood loss (MABL) is a calculation that estimates how much blood loss a patient can tolerate during a procedure. It is calculated using the patient’s estimated blood volume and hematocrit, and is important to consider when planning anaesthesia for procedures that may result in significant blood loss. MABL is calculated to help prevent unnecessary blood transfusions and under-resuscitation. It is particularly important in small children and neonates.
Blood loss below the MABL: replaced with crystalloid or colloid to maintain intravascular volume.
Blood loss above the MABL: replaced with packed red blood cells to keep the hematocrit at or above the desired value
Equation
Maximum Allowable Bloos Loss = (Estimated Blood Volume x [Starting Hematocrit – Lowest Hematocrit])/Starting Hematocrit
Hemoglobin may be substituted for hematocrit in the formula.
Estimated Blood Volume (EBV) = body weight x average blood volume
| Age range | Average blood volume (ml/kg) |
|---|---|
| Preterm neonates | 95 (90 – 100) |
| Full term neonates | 85 (80 – 90) |
| Infants between 3 months – 1 year | 70 – 80 |
| Older children | 70 |
| Obese children | 60 – 65 |
Intra-operative Oliguria
- Pre-renal causes
- Hypovolemia
- Decreased cardiac output (LV dysfunciton, valvular disease)
- Decreased MAP
- Increased intra-abdominal pressure e.g laparoscopy and pneumoperitoneum
- Post-renal causes
- Kinked, clogged, displaced or disconnected foley catheter
- Surgical manipulation of the kidneys, ureters, bladder or urethra
- Renal causes
- Neuroendocrine response to surgery (activation of RAAS, increased ADH) – age dependent
- Baroreceptor response to PPV activates neuroendocrine response
- Treatment of intra-operative oliguria
- Relieve obstruction: check foley, consider IV dye to check for patency of ureters (in urology cases)
- Increase renal perfusion: fluid bolus vs maintenance rate, vasopressors, inotropes, furosemide
Catheters
French (Fr): outer diameter/0.33 in m. Roughly approximates the circumference in mm
French x 0.33 = Outer Diameter in mm
Gauge: refers to needle outer diameter, not directly correlated in mm
16 G ~ 1.65 mm ~ 5 Fr
- Options for Venous Access In decreasing order of Max speed of infusion
- Introducer (Cordis – Central line that is short and has a single wide lumen) – Very Fast
- Peripheral IV (14G – 16G)
- Triple Lumen Catheter (Central line)
| Gauge | Colour | Flow rate (ml/min) | Time to give 1L bolus | Outer Diameter (mm) |
|---|---|---|---|---|
| 14 G | Orange | 250-300 | 4 min | 2.1 |
| 16 G | Grey | 180 – 200 | 5.5 min | 1.65 |
| 18 G | Green | 75 – 120 | 11 min | 1.27 |
| 20 G | Pink | 40 – 80 | 17 min | 0.9 |
| 22 G | Blue | 36 – 55 | 28 min | 0.71 |
| 24 G | Yellow | 20 – 35 | 50 min | 0.56 |
Further Reading
- Dellinger RP, et al. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock. Intensive Care Med. 2013; 39: 165-228