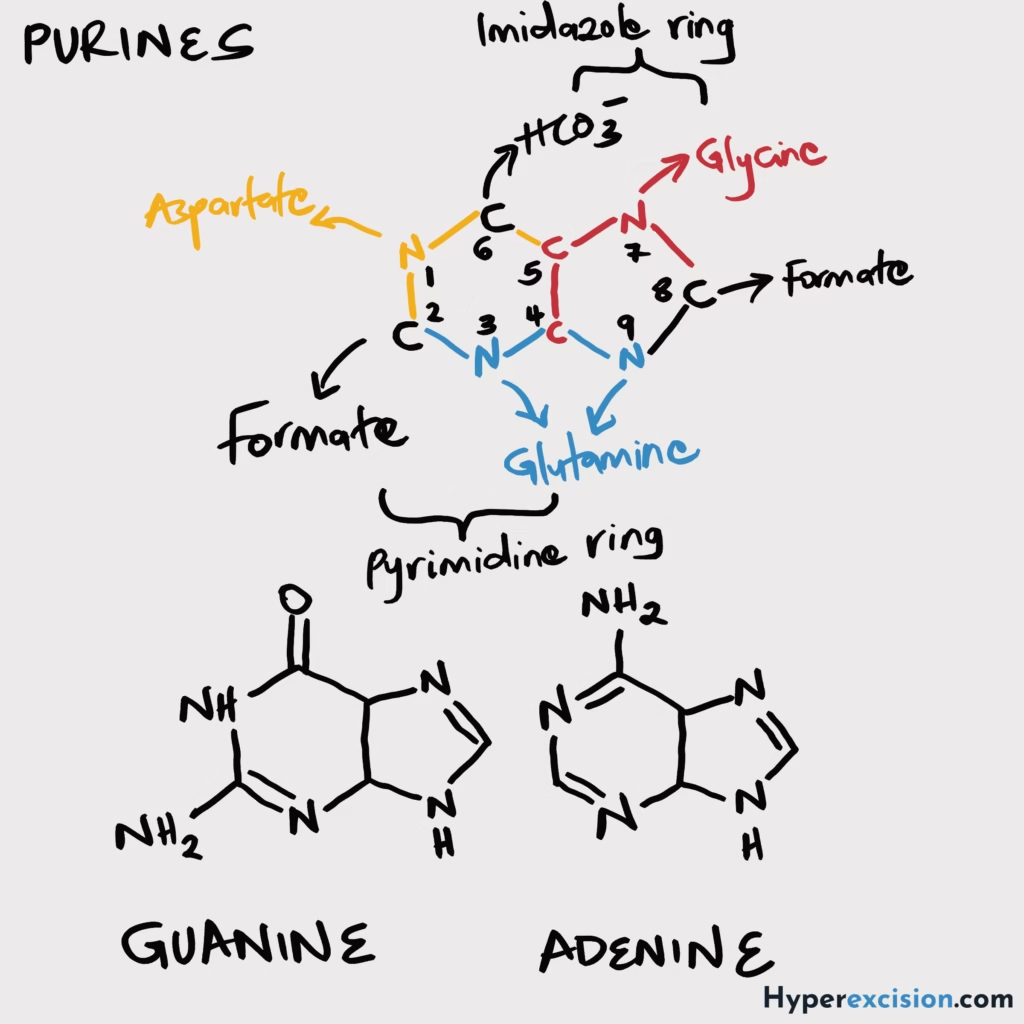
- Classify congenital immunodeficiency disorders
- Congenital B-cell immunodeficiencies
- Bruton agammaglobulinemia
- Selective IgA deficiency
- Common Variable Immunodeficiency
- Congenital T-cell immunodeficiencies
- DiGeorge Syndrome
- Autosomal dominant Hyper IgE syndrome (Job Syndrome)
- IL-12R deficiency
- Chronic mucocutaneous candidiasis
- IPEX syndrome
- Congenital combined immunodeficiencies
- Severe Combined Immunodeficiency
- Wiskott-Aldrich Syndrome (WAS)
- Hyper-IgM syndrome
- Ataxia Telangiectasia
- Congenital Neutrophil and Phagocyte disorders
- Chronic Granulomatous disease
- Leukocyte adhesion deficiency type 1
- Chediak-Higashi syndrome
- Myeloperoxidase deficiency
- Severe Congenital Neutropenia
- Complement disorders
- C1 esterase inhibitor deficiency
- Early complement deficiencies
- C1, C2, and C4 deficiency
- C3 deficiency
- Terminal complement deficiency
- Congenital B-cell immunodeficiencies
- Discuss the laboratory assessment of the adaptive immune system (B and T cells)
- Quantitative assay
- WBC and differential
- Absolute lymphocyte Count: Compare to age-matched controls
- Flow cytometry: Immunophenotyping of B and T cells, review percentage and absolute numbers and compare with age-appropriate ranges
- In vivo Qualitative B cell assays
- Serum Immunoglobulin concentrations: IgG, IgA, IgM and IgE classes compared with age-matched reference ranges, uses nephelometry or turbidimetry
- Immunoglobulin Sub-class levels: Investigated when class levels are below the age-matched reference ranges
- Serum Blood Grouping: anti-A or anti-B isohemagglutinin IgM levels, titre of 8 is normal
- Vaccine induced antibody levels: antibodies to previous immunization (pneumovax, HiB) measured 2-4 weeks post-vaccination
- In vitro Qualitative T or B cell assay
- Lymphocytic Proliferation Assay (LPA): Proliferative response to mitogens, Whole blood or Purified peripheral blood mononuclear cells (PBMC) are cultured with mitogens for 3-7 days and cellular proliferation or antibody secretion is measured
- Cytokine Production assay: Done using flow-cytometry of serum or culture
- Quantitative assay
- Discuss the laboratory assessment of complement function
- CH 50
- Classical pathway
- Sheep RBC sensitized with anti-sheep rabbit IgG are incubated with serial dilutions of patient’s serum
- Activation of classical pathway causing C3b and MAC deposition on sheep RBC resulting in hemolysis
- Hemoglobin is released into the supernatant and measured at 540 nm. Results measured as % lysis
- 50% lysis is obtained at a certain dilution and compared to control e.g. Control of 1:50 dilution vs sample 1:25
- Reduced CH 50: C1 (q,r,s), C2, C3, C4, C5-9, C1 INH, Factor I and H abnormalities
- AH 50
- Alternate pathway
- Rabbit RBC used because they have low sialic acid → cannot bind factor H → not protected from activation of alternate pathway
- Rabbit RBCs incubated with patient serum
- Activation of alternate pathway, deposition of C3b and MAC results in hemolysis, release of hemoglobin and measurement as % lysis at 540nm
- Reduced AH 50: C3, factor B, Factor D, C5-9, Soluble regulatory proteins (Factor H, I and properdin) activity
- C2, C3 and C4 protein levels
- To establish which complement protein is deficient
- ELISA to detect LP function
- Patient’s serum is placed into wells coated with Mannan
- Detects MBL levels
- Can also be detected antigenically
- CH 50
- Discuss the laboratory assessment of phagocyte dysfunction
- Screening studies
- CBC
- Cyclic neutropenia = ANC 2-3 times a week for at least 4-6 weeks
- Kostmann syndrome (Severe congenital neutropenia) = ANC <0.5 x 10^9/l several occasions
- PBF: Phagocyte number and morphology
- CBC
- BMA: Exclude aplasia due to malignancy or other causes, document other abnormalities IE. maturation arrest in Kostmann syndrome
- Functional Assays for Neutrophils
- LAD-1: Flow cytometry CD11 and CD18
- LAD-2: Flow cytometry CD15 (Sialyl-Lewis X)
- Chronic Granulomatous disease
- Nitroblue tetrazolium test: Yellow = abnormal
- Dihydrohodamine 123 assay: Abnormal
- Screening studies
- What is the association between complement and Disease
- C1 deficiency: Recurrent sinopulmonary infections (S. pneumo)
- C3 deficiency: Recurrent severe childhood infections with encapsulated bacteria (SHiN)
- Terminal deficiency: Increased risk of infections with Neisseria (Meningococcus)
- Terminal deficiency: reccurent neisserial infections
- C3nef: glomerulonephritis
- C1 esterase inhibitor deficiency: hereditary angioedema
- Briefly describe Properdin deficiency
- X-linked
- Alternative pathway deficiency – inability to stabilize C3 and C5 convertase
- Briefly describe C1 deficiencies
- Decreased or abnormal (LMW) C1q, Decreased C1r, Decreased C1s
- Inability to form the C1 complex
- Disruption of classical pathway (IgM and IgG dependent)
- What are the laboratory features of Factor D deficiency, Factor B deficiency, and Properdin deficiency lab
- Normal CH50
- Low AH50 (<10%)
- What are the laboratory features of C1, C2, C4, C1 INH deficiency lab
- Low CH50 (<10%)
- Normal AH50
- What are the laboratory features of C3 deficiency, C5-C9 deficiency, Factor H deficiency, Factor I deficiency
- Low CH50 (<10%)
- Low AH50 (<10%)
- Describe the pathogenesis of Chediak-Higashi Syndrome
- AR mutation in LYST gene (Lysosomal Trafficking Regulator gene)
- Affects: Leucocytes, melanocytes, platelets
- Defective neutrophil chemotaxis
- Defective microtubule polymerization → Defective phagosome-lysosome fusion
- Also defective melanosome = albinism
- Outline the laboratory features of Chediak-Higashi Syndrome
- CBC: Pancytopenia, especially neutropenia
- PBF: Giant cytoplasmic granules in granulocytes and platelets
- Mild coagulation abnormalities
- Describe the pathogenesis of Ataxia Telangiectasia
- ATM gene mutation
- defective dsDNA breaks repair
- Mutations accumulate
- Can lead to tumorigenesis (leukemia, lymphoma or gastric carcinoma)
- Apoptosis of B and T cells (Talk about P53 and RB)
- B and T cell deficiency
- Describe the pathogenesis of Wiskott-Aldrich Syndrome
- X-linked mutated WASp gene
- Impaired signalling and
- Impaired actin polymerization and cytoskeletal reorganization
- Failed formation of immunological synapses
- Defective antigen presentation
- Defective antibody dependent cell-cytotoxicity
- Defective phagocytosis
- Defective Treg function – autoimmune
- Failed formation of immunological synapses
- Briefly describe Chronic Granulomatous disease
- Caused by a defect in NADPH oxidase – required for the generation of peroxides and superoxides
- two types:
- X linked- NADPH oxidase- membrane component
- AR- NADPH oxidase- cytoplasmic component
- Due to defective neutrophil killing,
- There is formation of granulomas. the granulomas may cause obstruction of lumen eg GIT
- Individuals are susceptible to Bukholderia and Serratia
- lab investigation- nitroblue tetrazolium test
- Classic features of Wiskott-Aldrich Syndrome WATER
- Easy bruising and bleeding (Thrombocytopenia)
- Recurrent infections (Immunodeficiency)
- Eczema (idk)
- Outline the laboratory features of Wiskott-Aldrich Syndrome
- Normal or decreased IgG and IgM
- Elevated IgE and IgA (IgA is significantly increased) wAtEr syndrome
- CBC: Thrombocytopenia
- PBF: Small platelets
- Genetic analysis: Mutated WASp gene
- List the genes involved in **Severe Combined Immunodeficiency (**SCID)
- X-linked
- IL-2R
- IL-7R
- Autosomal Recessive
- ADA
- RAG
- JAK3
- MHC
- PNP
- X-linked
- Briefly describe the pathogenesis of SCID
- X-linked recessive
- Mutation in gene encoding common gamma chain
- Defective IL-2Ry chain linked to JAK3
- Autosomal recessive
- ADA deficiency
- Accumulation of deoxyadenosine and dATP and Disrupted purine salvage
- Inhibition of ribonucleotide reductase by dATP
- Others
- JAK3 deficiency -IL-2 signals via JAK/STAT
- RAG mutation → defective VDJ recombination
- X-linked recessive
- What are the clinical features of SCID
- Severe bacterial and viral infections
- Chronic diarrhea
- Mucocutaneous candidiasis
- What are the laboratory features of SCID
- Decreased T cells
- Decreased B cells
- Decreased NK cells
- Decreased antibody
- Briefly describe the pathogenesis of Bruton’s Agammaglobulinemia
- X-linked mutation in BTK → fail to pass checkpoint at pro-B cell → defective maturation → complete maturation of mature B cells
- What are the laboratory features of Bruton Agammaglobulinemia
- Flow: low CD19, CD20, CD21; Normal T cells
- Low Igs of all classes
- Absent lymphoid tissue (Germinal centres and primary follicles)
- Briefly describe the pathogenesis of **Leucocyte Adhesion Deficiency Type 1 (**LAD1)
- AR → absence of B2 integrin Leukocyte adhesion surface molecule LFA1 (CD18_ → leukocytes cannot migrate to tissue during infection or inflammation
- LAD2 is associated with Sialyl-lewis x
- What are the clinical features of LAD1
- Recurrent bacterial infections
- Impaired wound healing
- Omphalitis
- Delayed separation of the umbilical cord >30 days post-partum
- What are the laboratory features of LAD1
- Flow absent cd18, cd11a, cd11b, cd11c
- Leucocytosis in CBC
- Briefly describe Hyper-IgM syndrome
- X linked (Xq26) mutation in gene for CD40L on T-cells.
- CD 40L is important for costimulation and activation of macrophages and dendritic cells.
- no costimulation= no B cell activation = no class switching
- no activation of macrophages and dendritic cells = infection with P.jiroveci
- there will be an excess of IgM and lack of IgG,IgA and IgE
- IgM will cause destruction of blood cells (autoimmune hemolytic anaemia, thrombocytopenia and neutropenia)
- mutation-70% are Xq26. 30% are AR mutation of activation induced cytidine deaminase (AID), a DNA editing enzyme