Myelodysplastic syndromes are characterized by dysplastic and ineffective hematopoiesis – bone marrow failure**.** It is sometimes described as “pre-leukemic” since there is a risk of transformation into acute leukemia (30%). In MDS, there is varied anemia, thrombocytopenia, and leukopenia. Hence there is a risk of life-threatening infection and bleeding. Patients require multiple blood transfusions in the form of pRBCs and platelets.
Most patients are > 70 years old.
Myeloproliferative neoplasms (MPN) vs. myelodysplastic Syndrome (MDS) vs. acute Myelogenous Leukemia (AML)
| Features | MPN | MDS | MDS/MPN | AML |
|---|---|---|---|---|
| Bone Marrow Cellularity | Increased | Usually increased | Usually increased | Usually increased |
| % Marrow blasts | Normal or <10% | Normal or <20% | Normal or <20% | Minimal |
| Maturation | Present | Present | Present | Minimal |
| Morphology | Normal | Abnormal | Abnormal | Dysplasia can be present |
| Haematopoiesis | Effective | Ineffective | Effective or ineffective | Ineffective |
| Blood counts | One or more myeloid increased | Low, one or more cytopenia | Variable | Variable |
| Hepatosplenomegaly | Common | Uncommon | Common | Uncommon |
- WHO Classification of Myelodysplastic Syndromes (MDS)
- MDS with single lineage dysplasia (MDS-SLD)
- Dysplasia in >10% of one cell line, 1 or 2 blood cytopenias, <5% blasts
- MDS with multiple lineage dysplasia (MDS-MLD)
- >10% dysplasia in 2 or more lineages, 1-3 blood cytopenias, <5% blasts, <15% ring sideroblasts
- MDS with ring sideroblasts (MDS-RS)
- MDS with ring sideroblasts and single lineage dysplasia (MDS-RS-SLD)
- MDS with ring sideroblasts and multiple lineage dysplasia (MDS-RS-MLD)
- Anemia, No blasts, >15% of erythroid precursors with sideroblasts, Dysplasia in one or more cell lines
- MDS with isolated del (5q)
- MDS with excess blasts (MDS-EB)
- MDS unclassifiable (MDS-U)
- With 1% blasts
- With single lineage dysplasia and pancytopenia
- Based on defining cytogenetic abnormality
- Refractory cytopenia of childhood
- MDS with single lineage dysplasia (MDS-SLD)
- Causes of MDS ***Unclear, but there is a stepwise acquisition of oncogenic mutations
- de novo mutations
- Chemotherapy (alkylating agents)
- Environmental toxins (benzene)
- Radiation (therapeutic or accidental)
- Risk factors for MDS
- Genetic
- Constitutional genetic disorders: Down Syndrome, Trisomy 8, Mosaicism, Monosomy 5, Monosomy 7, Del 5q
- NFT1
- Germ cell tumors (embryonal dysgenesis),
- Congenital neutropenia: Kostmann’s or Schwachmnan-Diamond syndrome
- DNA repair deficiencies: Fanconi anemia, Ataxia telangiectasia, Bloom syndrome, Xeroderma pigmentosum
- Mutagen-detoxification (GSTQ1-null)
- Acquired
- Old age
- Cytotoxic therapy: Alkylating agents, Topoisomerase II inhibitors, Beta-emitters such as radioactive p-32,
- Hematopoietic cell transplantation;
- Environmental and occupational toxins: Benzene, Tobacco use
- Aplastic anemia
- Paroxysmal nocturnal hemoglobinuria (PNH),
- Polycythemia vera
- Obesity
- Genetic
- Signs and symptoms
- Fatigue, pallor, breathlessness (due to anemia)
- Severe and/or frequent infections (due to neutropenia0
- Easy bruising and bleeding (due to thrombocytopenia)
- Investigation
- Complete Blood Count – Cytopenias
- Macrocytic or normocytic anemia
- Low reticulocyte count
- Neutropenia
- Thrombocytopenia (Variable)
- Pancytopenia (50% of cases at presentation)
- Reticulocyte count – decreased
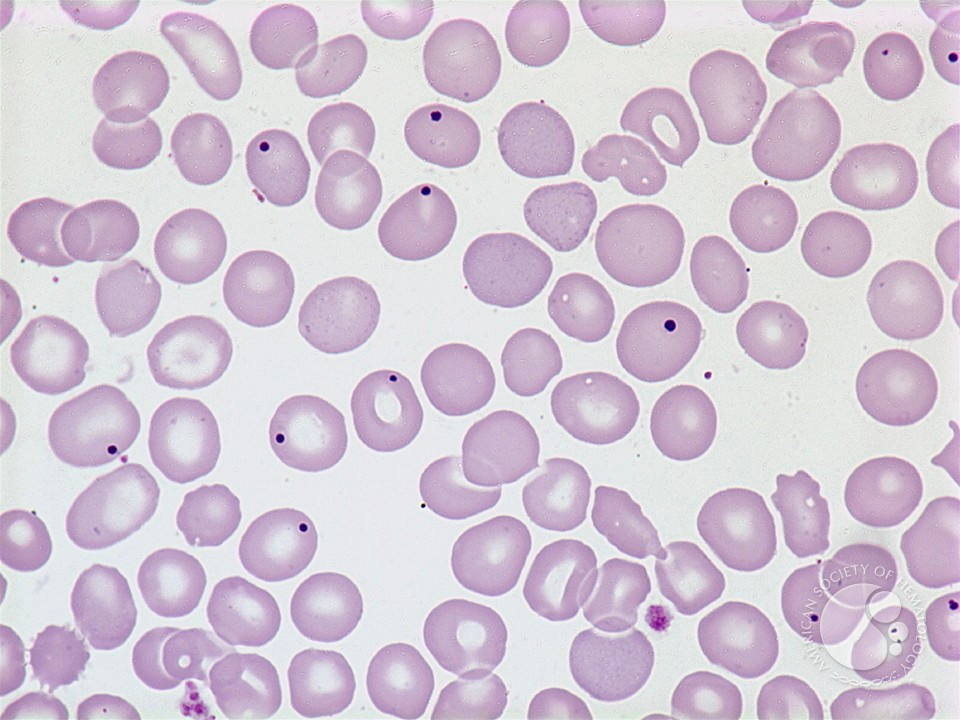
- Peripheral blood film – Cytopenias in peripheral blood
- Erythroid: Ovalomacrocytes, elliptocytes, acanthocytes, stomatocytes, teardrops, nRBC, basophilic stippling, Howell-Jolly bodies
- Myeloid: Pseudo Pelger-Huet anomaly, Auer rods, Hypogranulation, nuclear stick, hypersegmented, ringed-shaped nuclei, very coarse granules
- Megakaryocytes: Giant platelets, hypogranular or agranular platelets
- Bone marrow Studies – Dysplasia of all nonlymphoid lineages associated with cytopenia, ineffective hematopoiesis
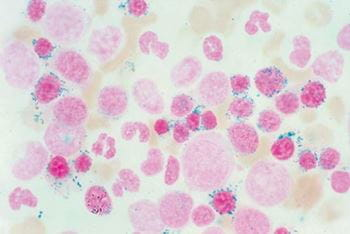
- Erythroid: Megaloblastoid erythropoiesis, Nuclear budding, Ringed sideroblasts, internuclear bridging, Karyorrhexis, nuclear fragments, cytoplasmic vacuolization, multinucleation – dysplastic changes in erythroid precursors with megaloblastic changes and presence of ringed sideroblast in iron stain
- Myeloid: Defective granulation, maturation arrest at myelocyte stage, increase in monocytoid forms, abnormal localization of immature precursors – Hyperplasia with dysgranulopoiesis
- Megakaryocytes: Micromegakaryocytes, Large mononuclear forms, hypogranulation, multiple small nuclei – Dysmegakaryopoiesis – pawn ball megakaryocytes
- Iron stores: increased with ring sideroblasts
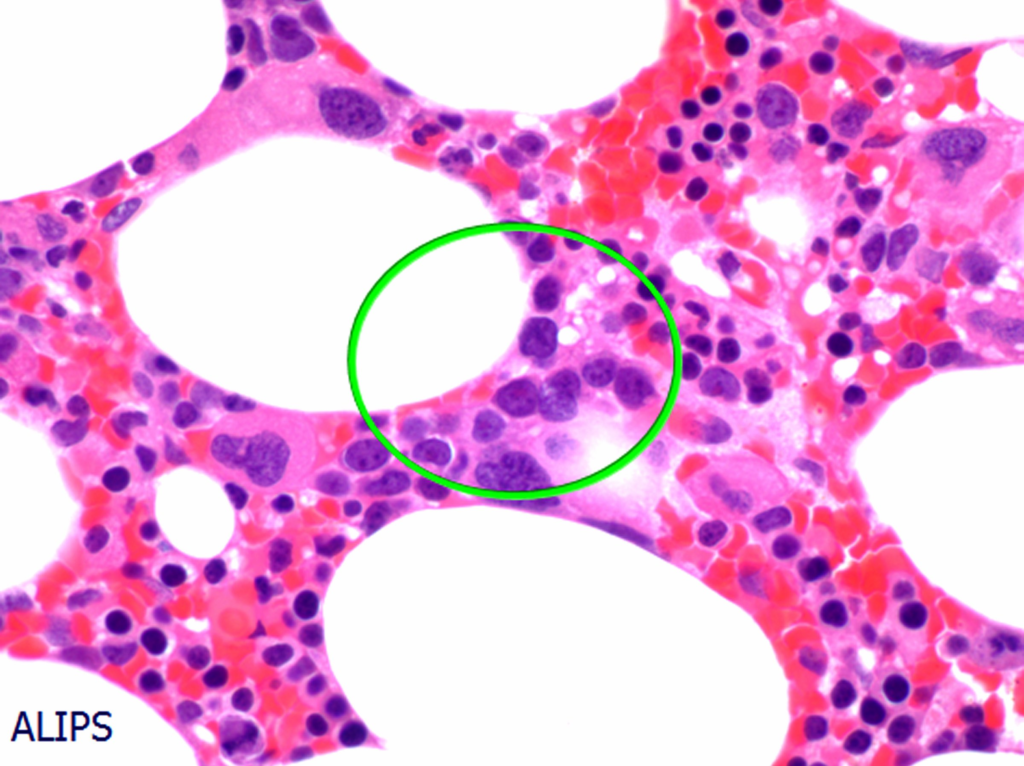
- Refractory anemia with excess blasts (RAEB) – Abnormal localization of immature precursors (ALIP)
- Cytochemistry
- Iron staining (Perls-prussian reaction) to ID ringed sideroblasts
- PAS staining of erythroblasts to assess dyserythropoiesis
- Peroxidase or Sudan Black B to detect myeloid lineage of blasts
- NSE or CAE to assess myeloid blasts
- Immunocytochemistry and Flow cytometry
- Exclude lymphoid origin of primitive blasts
- Distinguish Erythroid precursors: glycophorin (CD235a) or transferrin receptor (cd71)
- Quantify Myeloblasts: CD34, CD117, CD13, CD14, CD33
- Detect dysplastic or immature Megakaryocytes: vWF, CD41, CD61, HPI-ID monoclonal antibody
- Detect lineage infidelity
- Genetics/ Molecular (TR-PCR, FISH, Karyotyping)
- Detection of chromosomal abnormalities by RT-PCR, FISH (Distinguished between MDS and AML, aids in the classification of MDS, Major factor in determining prognostic risk group and therapy)
- Constitutional genetic disorders: Down Syndrome, Trisomy 8, Mosaics, Monosomy 5, Monosomy 7, Del 5q
- Other syndromes: NFT1, Germ cell tumors, Kostmann’s, Schwachman-Diamond syndrome, Fanconi anemia, Ataxia telangiectasia, Bloom syndrome, Xeroderma pigmentosum etc.
- Detection of chromosomal abnormalities by RT-PCR, FISH (Distinguished between MDS and AML, aids in the classification of MDS, Major factor in determining prognostic risk group and therapy)
- Complete Blood Count – Cytopenias
- What is meant by Abnormal Localization of Immature Precursors (ALIP) in MDS?
- ALIP refers to the ****localization of myeloblasts and promyelocytes (immature precursors) in the intertrabecular region in abnormal clusters instead of the endosteum where they are normally found.
- It is commonly seen in MDS refractory anemia with excess blasts (RAEB)
- Treatment of MDS
- Multiple transfusions – pRBCs and Platelets
- Erythropoietin +/- G-CSF
- Bone marrow transplant: curative but inappropriate since most patients are > 70 years
- Thalidomide analogues (Lenalidomide) or hypomethylating agents (azacitidine and decitabine) may improve quality of life