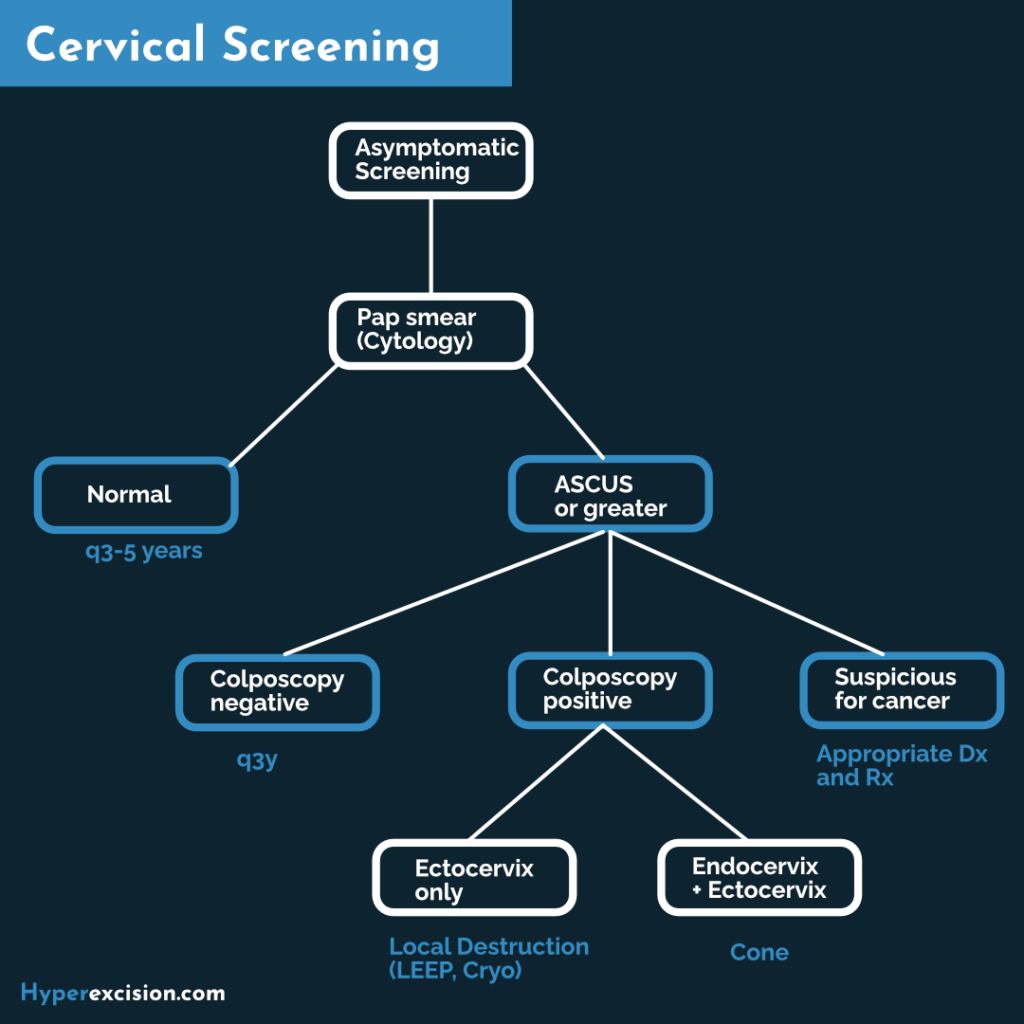
Overview
Cervical screening is one of the cornerstones of gynaecological visits. Every woman attending a clinical visit should have their records assessed to ensure they are up to date on their cervical screening. HPV DNA testing is not recommended before the age of 30 because many young women are infected with HPV, most of which will spontaneously resolve at 30 years.
The morbidity and mortality of cervical cancer have drastically reduced since screening began. Cervical dysplasia and cervical cancer are triggered by HPV. Men can carry virulent strains of HPV and pass it on to their partners. How many sexual partners the man has had matters just as much as the woman. Vaccination is very important. Young boys can also be given the vaccine so that they do not become asymptomatic carriers.
Cervical cancer is the third most common malignancy worldwide. In developing countries, it is the second most common cause of cancer deaths. The incidence in Eastern Africa is 34.5 cases per 100,000. The median age of diagnosis is 52 years. Cervical intraepithelial neoplasia is commonly diagnosed in the 20s. Carcinoma in Situ is commonly diagnosed at 35-45y. It takes time (approximately 10 years) for dysplasia to progress to carcinoma.
- Screening methods for cervical cancer
- Pap smear: primary screening in these situations:
- Primary screening for women < 30 years (starting at 21 years)
- HPV testing is unavailable
- Women not eligible for VIA/VILI because the SCJ is not visible
- Co-test with HPV testing in HIV-positive women if available
- HPV testing: primary screening for women > 30 years
- VIA/VILI: primary screening where HPV testing is unavailable or loss-to-follow-up is a risk
- Pap smear: primary screening in these situations:
- Who should be screened for cervical cancer?
- Any woman who has ever had sexual intercourse
- Women aged 25-49 years
- Women aged 50-65 years are still at risk and can receive screening q5y
- How often should cervical cancer be screened for?
- q5y in women who test negative for HIV, upto 65 years of age
- q1y if VIA/VILI or Pap smear, or q2y if HPV testing for women who are HIV positive or immunosuppressed
- First trimester in pregnant women
- Can continue from 6 weeks post-partum
- Screening should be discontinued for women who have had Total Abdominal Hysterectomy
The Pap Smear
Georgios Papanikolaou found out that cellular debris can give clues to cancerous processes and the stage of the menstrual cycle the woman is in. In 1928, he presented his findings that there were consistent differences in the cells of women who later on developed cervical cancer to those who didn’t. By 1970 pap smears were recommended as routine screening for women.
- When to Pap smear (ACOG)
- First Pap smear at age 21 (cytology)
- Subsequent Pap smears q3y until 30 years
- Past 30 years routine Pap smears q3y. If HPV testing is added to the regular pap it may be done q5y
- When to discontinue Pap smears (ACOG) Women with significant risk factors for cervical cancer should be discouraged from discontinuing Pap smears
- From age 65 pap smears may be discontinued if:
- Patient has never had CIN 2+
- Patient is more than 20 years removed from the treatment of CIN2+
- Total hysterectomy performed for reasons other than cancer provided there was no abnormal pap in the past 20 years
- From age 65 pap smears may be discontinued if:



Abnormal cytology from Pap smears
Pap smears are read by a clinical cytopathologist who gives descriptors of abnormal cytology. With the exception of ASC-US, all abnormal pap smears in women > 24 years proceed to colposcopy and biopsy. If the test is positive for high-risk HPV she should get colposcopy (VIA/VILI) and biopsy. Otherwise, pap and HPV DNA may be repeated in three years.
| Grading acronym | Description |
|---|---|
| ASC-US | Atypical squamous cells of undetermined significance |
| LSIL | Low-grade squamous intraepithelial lesion |
| ASC-H | Atypical squamous cells, cannot exclude high-grade SIL |
| HSIL | High-grade squamous intraepithelial lesion |
| AGC | Atypical glandular cells |
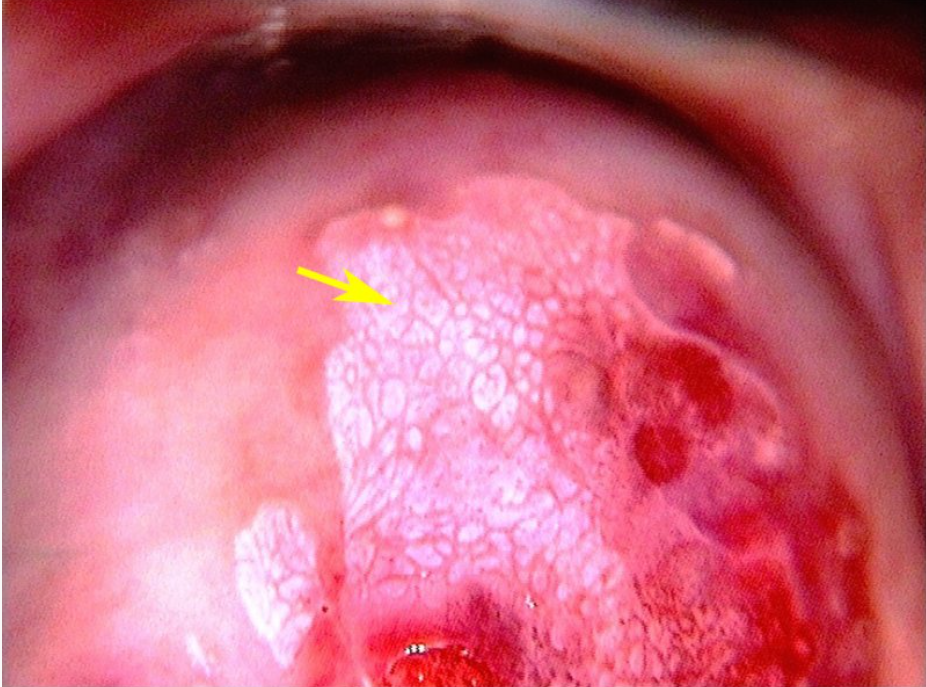
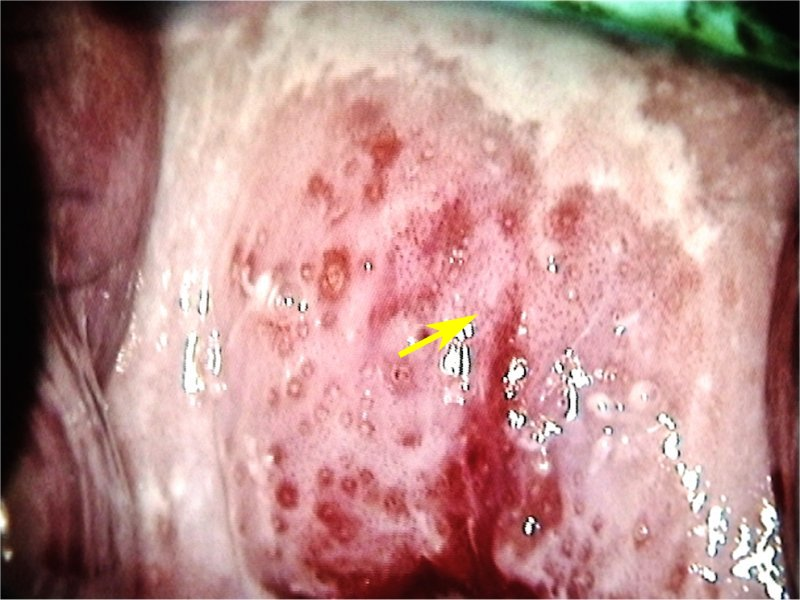
Colposcopy/Visual Inspection with Acetic Acid (VIA)
Morphological changes in the cervix and vagina can show where dysplasia is located. Abnormal areas are biopsied. The transformation zone is the most common area for dysplasia to occur
- What are you looking for?
- Areas that stain white with acetic acid (Acetowhite areas)
- Areas that are “mosaic”-like in appearance (”Cobblestoning”)
- Areas that display punctated vessels
- Areas with abnormal vessel geography (forming bizarre shapes, etc. Vessels should arborize, like the branches of a tree)
- Who cannot get VIA?
- Women who are very ill
- Women in the 2nd and 3rd trimester of pregnancy
- Women < 6 weeks after delivery
- Women with cauliflower-like growth or ulcer (fungating mass)
- Women with previous history of treatment of cancerous lesions
- Women with known allergy to acetic acid
- Women with a history of total hysterectomy
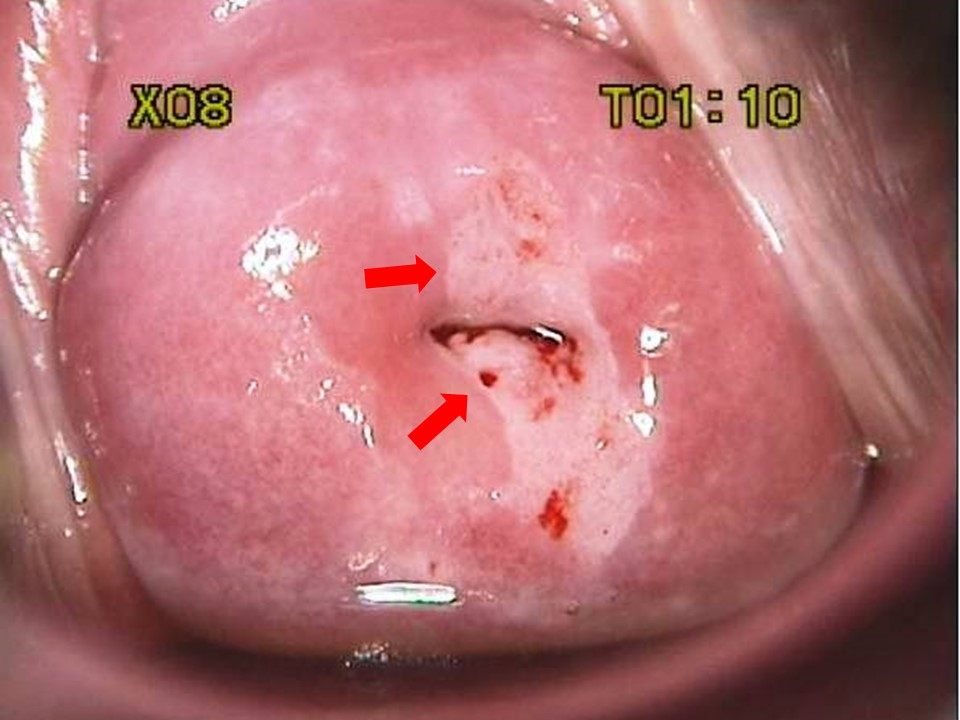
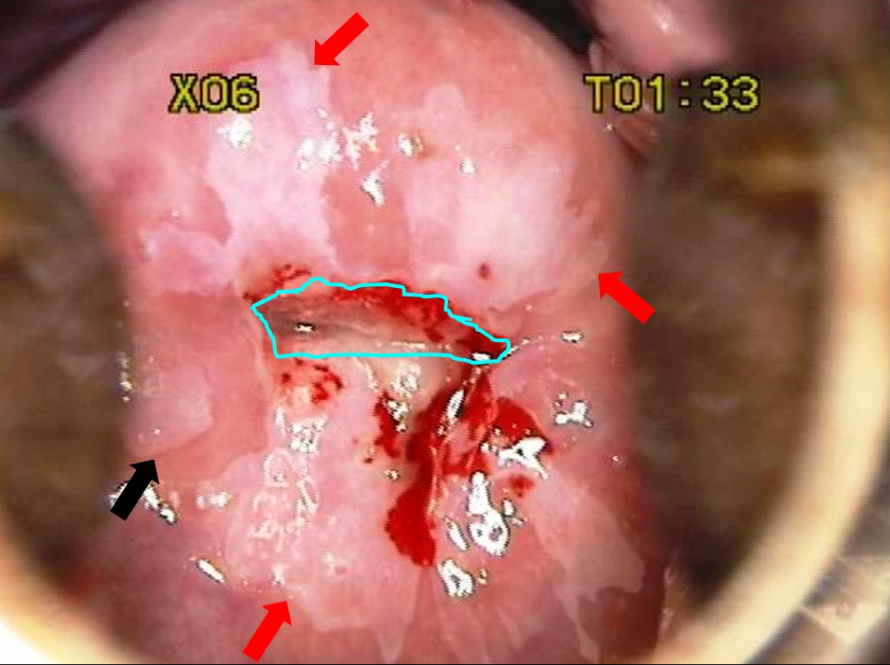
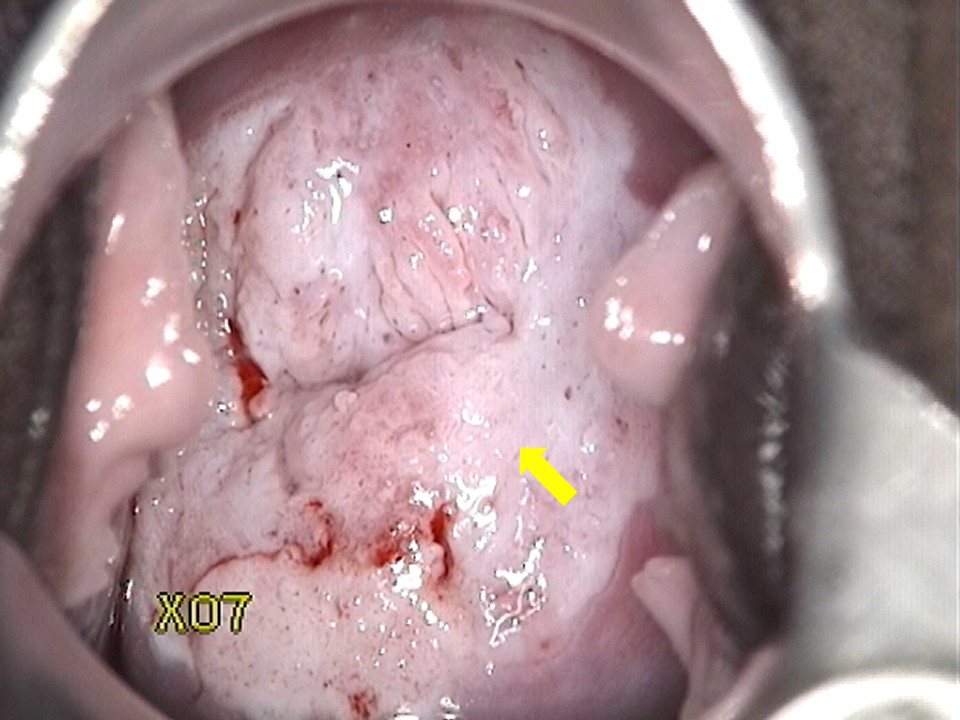

Histology
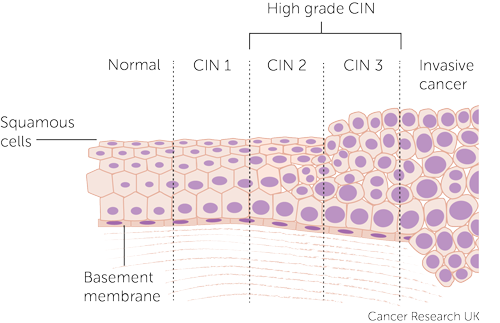
Histopathologists assess the biopsy and report it based on the depth of abnormal cellular invasion and whether or not the basement membrane is intact
| CIN 1 | Dysplasia restricted to deep 1/3 of the epithelium |
|---|---|
| CIN 2 | Dysplasia approximately 2/3 of the epithelium |
| CIN 3 | Dysplasia more than 2/3 of the epithelium |
| CIS | Full thickness dysplasia |
| Invasive cervical cancer | Penetrates the basement membrane |
Management of Abnormal Cervical Cytology and Histology
Histologic diagnosis is important for determining the therapeutic intervention, prognosis, and surveillance.
- Management of CIN 1 Few will progress to invasive cancer (<1%)
- Pap smear + HPV in one year
- Abnormal result: Repeat colposcopy and biopsy
- Normal result (Pap and HPV): Return to annual pap smear
- Pap smear + HPV in one year
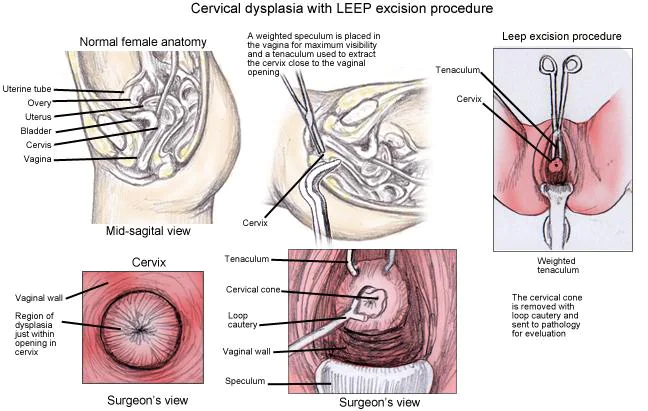
- Management of CIN 2, CIN 3, and CIS
- Surgical excision (Cervical conization)
- Loop electrical excision procedure (LEEP) or Large loop excision of the transformation zone (LLETZ)
- Cryosurgery
- Cold Knife Conization (CKC) – using a scalpel
- Surgical excision (Cervical conization)
Natural History of CIN lesions
| Spontaneous regression | Persists | Progress to CIN-III | Progress to invasive cancer | |
|---|---|---|---|---|
| CIN 1 | 60% | 30% | 10% | < 1% |
| CIN 2 | 40% | 35% | 20% | 5% |
| CIN 3 | 30% | 50% | N/A | 12-22% |

